New Delhi: The Medical Technology Association of India in its recently issued statement has argued that an unseen casualty of the price control could be the Foreign Direct Investment (FDI).
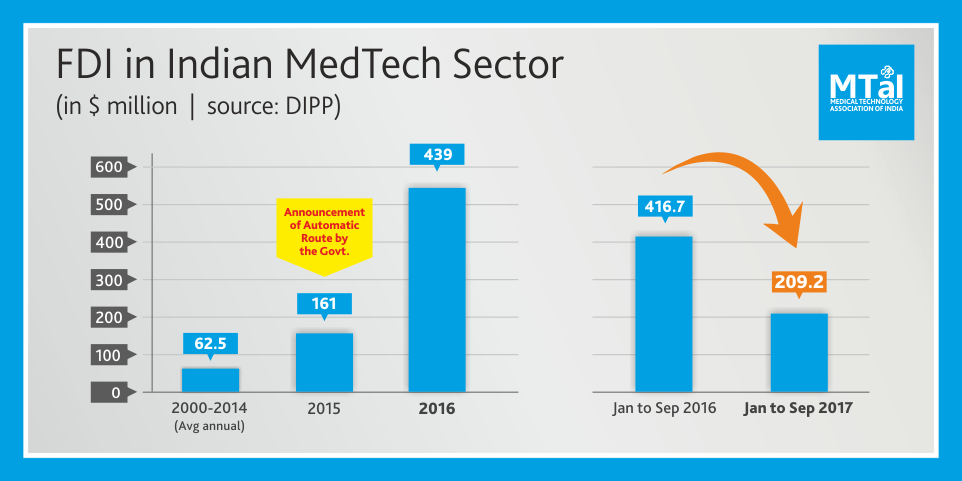
“This government took a pioneering step by bringing the FDI in medical devices on automatic route from February 2015. FDI rose from an average USD 63 million per annum to USD 161 million in 2015 and USD 439 million in 2016. The steady surge in FDI, since the decision to bring it on the automatic route is demonstrative of what improving ease of doing business for the relevant communities can accomplish, MTaI ‘s statement adding that “However, this year Jan-September 2017 figure of USD 209 million (extrapolated) shows a sharp fall against the figure of USD 417 million for the same period last year.”
The statement goes on to ask, “Could it be because of Market Shrinking measures like price control effected in February 2017?”